|
published in: "The Journal
of the Orders and Medals Society of America", volume 51,
number 4, pages 30-32, and received the
OMSA
Literary Medal 2001.
The
collecting and study of orders, decorations, and medals
is an interdisciplinary enterprise that requires some
knowledge of history, art and numismatics pertaining to
fakes is especially important. Some fakes are easy to
spot, but others are so deceptive only an expert on the
award in question can see through the fraud. Among the
most deceptive fakes are those produced by the
electroplating process; however, these fakes can often
be detected by collectors who have no particular award
expertise but do know how they were made.
Illustrated are the first
and second issues of the Nassau Life-Saving Medal. The
first issue was awarded on 13 February 1843 by Nassau’s
Duke Adolph. This silver medal, which is 30mm in
diameter, was designed by Christian Zollmann. Is It was
not meant to be worn. On the obverse is a large profile
head of a young Duke Adolph facing the right, and
encircling the profile is the inscription ADOLPH
HERZOG ZU NASSAU in raised letters. The reverse is
plain except for the inscription FUR/RETTUNG/AUS/LEBENSGEFHR
(literally, “For Rescue Out of Life-Threatening
Danger”) in the bottom. A total of 54 first-issue medals
were struck and awarded.
In 1865, 30 silver
life-saving medals of a new design and also 30mm in
diameter we struck. The 1843 reverse was retained, but
the obverse now featured the profile of an older looking
Duke Adolph facing to the left, and the name of the die
sinker, “KORN,” was added at the base of the
profile in small raided letters. Moreover, the new
design incorporated a suspension device and solid red
ribbon so that the medal could be worn. Only 5 of the
second issue were awarded, and the remaining 25 medals
were melted down in late 1866.
Thus both issues, and in
particular the second issue, are extremely rare and
highly prized by collectors of the awards of the German
states. Small wonder then that someone took the effort
to make the illustrated second issue, which is a fake
produced by electroplating.
Before closely examining
this fake, it is first necessary to briefly describe the
electroplating process. The principle was discovered by
Faraday in 1833, and the process was patented in England
in 1840. The purpose of electroplating is to transfer
and bind the atoms of one metal to the surface of
another metal or to the surface of an object coated with
a conductive material. Electroplating requires a DC
power supply, often a battery, a chemical bath called
the “electrolyte,” and an “anode” or positive pole at
one end of the bath and a “cathode” or negative pole at
the opposite end. When the anode is connected to the
positive pole of the power supply and the cathode to the
negative pole of the supply, electrons stream from the
metal of the anode to the cathode. This stream is part
of the electrical current that flows from the positive
pole of the power supply to the anode, through the
electrolyte to the cathode, and then from the cathode to
the negative pole of the power supply, thus completing
the circuit. Electricity is merely a stream of electrons
through a conductor, which can be either a metal wire or
the chemicals in a plating bath.
The shedding of electrons
from the anode simultaneously produces positively
charged atoms or “ions” of the metal of the anode. The
ions are attracted or pulled to an excess of electrons
gathering at the cathode. These ions bind with free
elections at the cathode producing neutral atoms of the
metal that attach to the cathode to form the plate. It
is important to note that the source of the plating
metal need not be the anode. Electroplating is often
accomplished by using a no reactive anode, such as
platinum, and an electrolyte containing a salt of the
plating metal. The chemistry is the same except that the
source of the plating metal is the electrolyte instead
of the anode. Of course, the chemical reaction is the
electroplating process is more complicated that
suggested by my simple explanation, and a number of
important technical aspects must be considered, such as
the duration of the process and the composition of the
electrolyte, to achieve the desired consistency and
thickness of the plate.
|
|
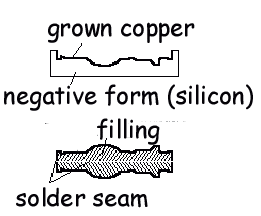
The
first step in producing a fake medal by
electroplating is to make an obverse and reverse
mold of the original. Early molds were made using a
mixture of bee’s wax and graphite, but today nearly
prefect molds can be achieved with the silicone
rubber compounds that are sued to make dental
impressions. After the mold is make, the inside is
covered with a conductible material, such as
graphite, and then the mold is attached to the
cathode in an electrolyte bath that has a copper
anode. When current is applied, a thin copper plate
or shell forms on the inside of the mold. Once the
obverse and reverse shells are make, the inside of
the shells are filled with a soft metal and the two
halves are soldered together. Copper is initially
used because it is less expensive than sliver, is
easy to plate, and has an extremely durable surface.
Later silver is electroplated to the assembled
copper shells. Silver plating usually involves the
use of a no reactive anode and an electrolyte
containing silver dissolved in nitric acid or a
cyanide solution. |
Electroplated fakes are
typically very difficult but not impossible to detect.
The detail can be perfect, and the surfaces are as
smooth as the original, unlike cast fakes which
typically have porous surfaces. In addition, a filler
can be used inside the shell that gives the fake the
same weight as the original. Nonetheless, the process
itself provides knowledgeable collectors with certain
indicators that can be used to determine whether or not
a particular medal has been produced by electroplating.
Consistent application of the following four indicators
should greatly aid collectors in avoiding the misery
brought by the purchase of one of these clever fakes:
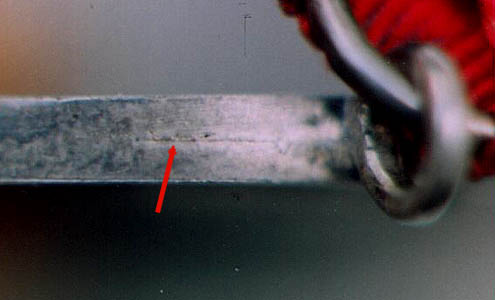
First and foremost, it
must be remembered that the fake was not make as one
piece but consists instead of two halves that were
soldered together. This means that despite the best
efforts of the counterfeiter to polish out he solder
seam, a portion of the tell-tale seam almost always
appears on the edge of the medal. That portion of the
seam visible on the edge of the fake second-issue Nassau
Life-Saving Medal is shown in the enlargement below.
Secondly, the fake
“sounds” different. Lead is often used to fill the
inside of the fake, and sometimes antimony is added to
the lead to give the fake the same weight as the
original. However, when tapped by a hard object, the
fake with its lead filler sounds, “dead” compared to a
solid silver piece, which has a clear metallic sound.
Thirdly, collectors
should carefully examine the medal for areas where the
silver plate has worn off and the copper plate
underneath shows through. Simply put, medals struck in
solid silver can’t have signs of copper.
Finally, one must also
carefully look at the medal’s detail. A fake produced by
electroplating can have flawless detail, but this in not
always the case because the surface of the fake is only
as good as the surface of the mold. Very often, there
are tiny deficiencies in the mold that appear in the
final product. In the next enlargement, several problems
are evident in the letters of the die sinker’s name on
the obverse of the fake second-issue Nassau Life-Saving
Medal.

Fakes produced by
electroplating are much harder to detect than cast
fakes, and counterfeiters have been greatly aided by a
lack of knowledge by both dealers and collectors of the
electroplating process and its indicators. To make
matters worse, electroplated fakes are appearing in the
market in increasing numbers. I remember from my days as
a young collector in Germany that German World War I
aviation badges were almost impossible to find. The
originals are still hard to find in Germany, but what is
new is that since the late 1980’s electroplated fakes
can be seen wherever awards are sold. The tide can only
be stemmed though education, and that is the intention
on this article.
© A. Schulze Ising,
VII/00
|





